Over the past year, both State and Federal law enforcement investigators and prosecutors have gone to considerable lengths to publicize the government’s fight against opioid abuse. While much of this fight has focused on the manufacturers of prescription opioid products, the improper prescribing practices of physicians and other medical professionals found to be prescribing these drugs “without any legitimate purpose and outside the usual course of professional practice” have been repeatedly highlighted in criminal prosecutions by U.S. Attorney’s Offices around the country. A recent case against a Philadelphia-area cardiologist provides a classic example of how improper opioid prescribing practices can lead to criminal prosecution, civil penalties AND severe administrative sanctions (in this case, exclusion from participation in Federal health care programs). The article examines how the cardiologists was identified and steps you should take to reduce your level of risk in this regard.
Over the past year, both State and Federal law enforcement investigators and prosecutors have gone to considerable lengths to publicize the government’s fight against opioid abuse. While much of this fight has focused on the manufacturers of prescription opioid products, the improper prescribing practices of physicians and other medical professionals found to be prescribing these drugs “without any legitimate purpose and outside the usual course of professional practice” have been repeatedly highlighted in criminal prosecutions by U.S. Attorney’s Offices around the country. A recent case against a Philadelphia-area cardiologist provides a classic example of how improper opioid prescribing practices can lead to criminal prosecution, civil penalties AND severe administrative sanctions (in this case, exclusion from participation in Federal health care programs). The article examines how the cardiologists was identified and steps you should take to reduce your level of risk in this regard.I. Background of the Case:
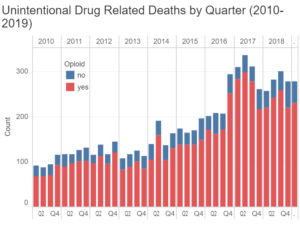
Like many big cities, Philadelphia has a problem with illegal drugs. Despite the city’s efforts to curb illicit drug use, unintentional drug overdoses have steadily grown since 2010 and have only slightly tapered-off from their all-time high since 2017.[1]

Are you unsure your screening requirements depending on your business and location? Our FREE Consultation has you covered. It includes: An overview of exclusions in addition to an overview of your specific requirements and obligations. Furthermore a demonstration of our product and service (SAFER) will be performed prior to a presentation of your personalized solutions. This consultation is a free of charge consultation for your benefit only!
While the percentage of opioid-related deaths changes from quarter to quarter, it is estimated that approximately 80% of all unintentional drug deaths in Philadelphia are due to opioid misuse and overdose. In response to the opioid abuse crisis in Philadelphia and other areas of the country, a multi-agency team of Federal and State investigators and prosecutors, known as the Medicare Fraud Strike Force[2] (Philadelphia Strike Force) has been targeting physicians, nurse practitioners and other medical professionals who have been improperly prescribing and / or distributing opioids. In this particular case, the prescribing practices of this Philadelphia cardiologist (defendant) were identified by the Philadelphia Strike Force as warranting further review. Upon investigation, the government alleged that from 2016 to 2018, the defendant wrote a number of prescriptions for oxycodone and / or benzodiazepine to patients without a legitimate medical purpose.
In light of the allegations presented, the U.S. Attorney’s Office pursued both civil and criminal claims against the cardiologist. It is important to keep in mind that the Department of Justice has long instructed its prosecutors to pursue parallel criminal and civil actions against a defendant, when appropriate. As Title 9, Section 27 of the Justice Manual[3] provides:
“Department policy is that criminal prosecutors and civil trial counsel should timely communicate, coordinate, and cooperate with one another and agency attorneys to the fullest extent appropriate to the case and permissible by law, whenever an alleged offense or violation of federal law gives rise to the potential for criminal, civil, regulatory, and/or agency administrative parallel (simultaneous or successive) proceedings. By working together in this way, the Department can better protect the government’s interests (including deterrence of future misconduct and restoration of program integrity) and secure the full range of the government’s remedies (including incarceration, fines, penalties, damages, restitution to victims, asset seizure, civil and criminal forfeiture, and exclusion and debarment).” (emphasis added).
“Courts have recognized that “[t]here is nothing improper about the government undertaking simultaneous criminal and civil investigations“ provided that we use those proceedings and associated investigative tools for their proper purposes and in appropriate ways.” (emphasis added).
II. Criminal Prosecution for Violations of 21 USC §841(a)(1) and (b)(1)(C):
In March 2019, the defendant pleaded guilty to eight felony counts of the unlawful distribution and dispensing of a controlled substance, in violation of 21 USC §841(a)(1) and (b)(1)(C). The defendant’s sentencing is scheduled to take place later this year. As a result of the criminal conviction, the defendant may be sentenced to prison for a significant period of time AND assessed criminal fines for his unlawful conduct.
III. Civil Liability under the False Claims Act, 31 USC §3729:
Notably, it does not appear that the defendant entered into a “global” settlement at the time he decided to plead guilty to the criminal charges discussed above. As a result, any civil and / or administrative actions that the government might choose to pursue remained active. On July 1, 2019, the defendant agreed to enter into a False Claims Act settlement with the government and pay $107,584 in penalties and damages to the government.
IV. Administrative Actions Taken Against the Defendant:
- Surrender his medical license and Drug Enforcement Administration Certificate of Registration and further agreed not to seek to
renew or reinstate either one in the future.- Be excluded from participation in Federal Health programs.
“Claims for excessive charges, unnecessary services or services which fail to meet professionally recognized standards of health care, or failure of an HMO to furnish medically necessary services.”
Under this statutory provision, an individual or entity can be excluded for a minimum period of one year. In light of the allegations presented, the defendant cardiologist was excluded from participation by HHS-OIG for a total of seven years.[4]
V. Impact of Medicare Exclusion on the Defendant’s Career:
As the case synopsis above reflects, Federal law enforcement prosecuted the defendant in this case to the full extent of their abilities. In addition to facing incarceration, the defendant was also assessed penalties and damages of more than $107,000 under the False Claims Act. While the criminal and civil actions taken against the defendant are quite serious, the cardiologist’s problems are further magnified by the fact that he has also been excluded from participation in Federal health care programs. At the end of the day, it is quite conceivable that the U.S. Sentencing Guidelines, the defendant’s criminal sentence will be relatively brief. Depending upon the terms of his civil / administrative settlement, he may be free to seek licensure in another state upon his release from jail.
VI. Final Thoughts:
Even assuming that the defendant regains licensure in another state, the administrative exclusion action taken against him will effectively bar him from enrolling in Federal health care benefit programs for the entire period that he remains excluded. While excluded, he will not be eligible to work for any provider or supplier who participates in one or more Federal health care plans. Should a health care provider or supplier choose to employ the defendant (an excluded party), each of the claims submitted to Medicare, Medicaid and other government plans may be subject to significant civil monetary penalties.
How can you protect your practice? Consistent with your obligations under the law, it is imperative that you screen your employees, agents, contractors and vendors against all of the 43 exclusion databases currently in operation. Unfortunately, it is practically impossible for a medical practice or other entity to screen one or more of its employees against all 43 databases. Luckily, the folks at Exclusion Screening can take this time-consuming (and often frustrating) task off of your shoulders.
Give us a call at 1 (800) 294-0952 for a complimentary discussion of your screening needs and a quote or fill out the form below!